The National Kidney Foundation’s 2024 Spring Clinical Meetings Late-Breaking Presentation on New Therapy Shows Promising Results for IgAN Patients
NEW YORK, May 14, 2024 /PRNewswire/ — During a late-breaking abstract presentation at the annual 2024 NKF Spring Clinical Meetings (SCM) tomorrow Dr. Dana Rizk, from the University of Alabama at Birmingham (UAB) Medical Center, will present data on the impact and safety of Fabhalta (iptacopan) in the treatment of patients with IgA nephropathy (IgAN) also known as Berger disease. Iptacopan was approved by the FDA in 2023 for the treatment of another disease entity, paroxysmal nocturnal hemoglobinuria. IgAN is an incurable glomerular disease that can cause chronic kidney disease (CKD) and is a leading cause of end-stage renal disease (ESRD). The presentation entitled, Efficacy and Safety of Iptacopan in Patients with IgAN: Interim Analysis (IA) of the Phase 3 APPLAUSE-IgAN Study will be held at the Long Beach Convention Center, 300 East Ocean Blvd, on Wednesday, May 15 from 2:30 to 4:30 p.m. (PT).
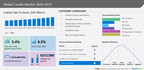
In the analysis, patients treated with iptacopan in addition to standard supportive care, achieved a 38.3% (p<0.0001) proteinuria reduction (as measured by 24-hour urine protein to creatinine ratio [UPCR]) at 9 months when compared to placebo1. Proteinuria reduction is an increasingly recognized surrogate marker correlating with progression to kidney failure and has been used in IgAN clinical trials to support accelerated approvals7. The study also showed that iptacopan was well tolerated by IgAN patients with a favorable safety profile consistent with previously reported data1,8.
Quote from Researcher
“In IgAN, part of the immune system called the alternative complement pathway can become overly activated in the kidneys, which causes an inflammatory response, leading to progressive kidney damage and gradual loss of kidney function. The loss of kidney function, together with potential side effects of IgAN treatments available until recently, significantly impact patients’ lives,” said Professor Dana Rizk, Investigator, APPLAUSE-IgAN Steering Committee Member, and Professor in the UAB Division of Nephrology. “Fabhalta is the first potential treatment for IgAN that specifically targets the alternative complement pathway.”
Highlights of the Study
APPLAUSE-IgAN is the first Phase III multicenter, randomized, placebo-controlled study to demonstrate significant proteinuria reduction by targeting the complement system in patients with IgAN. This pre-specified interim analysis included 250 patients for the efficacy analysis and 443 for the safety analysis1. The APPLAUSE-IgAN study continues in a double-blind fashion until the final analysis of the primary endpoint related to iptacopan’s ability to slow IgAN progression by measuring the annualized total estimated glomerular filtration rate (eGFR) slope over 24 months. Those results are expected at study completion in 20259,10.
The two primary endpoints of the study for the interim and final analysis, respectively, are proteinuria reduction at 9 months as measured by UPCR, and the annualized total eGFR slope over 24 months9,10. At the time of final analysis, the following secondary endpoints will also be assessed: the proportion of participants reaching UPCR <1 g/g without receiving corticosteroids/immunosuppressants or other newly approved drugs or initiating new background therapy for treatment of IgAN or initiating kidney replacement therapy (KRT), time from randomization to first occurrence of composite kidney failure endpoint event (reaching either sustained ≥30% decline in eGFR relative to baseline or sustained eGFR <15 mL/min/1.73 m2 or maintenance dialysis or receipt of kidney transplant or death from kidney failure), change from baseline to 9 months in the fatigue scale measured by the Functional Assessment Of Chronic Illness Therapy-Fatigue questionnaire9,10.
The main study population enrolled patients with an eGFR ≥30 mL/min/1.73 m2 and UPCR ≥1 g/g based on a 24 hr urine collection at baseline9,10. In addition, a smaller cohort of patients with severe renal impairment (eGFR 20–30 mL/min/1.73 m2 at baseline) was also enrolled to provide additional information but will not contribute to the main efficacy analyses9,10.
“There is a need for effective, targeted therapies for IgAN patients and the detailed Applause-IgAN study gives valuable, promising insights to healthcare providers and patients living with IgAN,” said NKF President Dr. Sylvia Rosas. “The alternative complement pathway has been implicated in the pathogenesis of IgAN, so it gives patients hope that a novel therapeutic intervention may lead to slowing progression of chronic kidney disease and avoiding kidney failure.”
The annual 2024 NKF Spring Clinical Meetings (SCM) will be held in Long Beach, CA from May 14 – 18.
NKF Spring Clinical Meetings
For the past 32 years, nephrology healthcare professionals from across the country have come to NKF’s Spring Clinical Meetings to learn about the newest developments related to all aspects of nephrology practice; network with colleagues; and present their research findings. The NKF Spring Clinical Meetings is designed for meaningful change in the multidisciplinary healthcare teams’ skills, performance, and patient health outcomes. It is the only conference of its kind that focuses on translating science into practice for the entire healthcare team.
About Kidney Disease
In the United States, more than 37 million adults are estimated to have kidney disease, also known as chronic kidney disease (CKD)—and approximately 90 percent don’t know they have it. About 1 in 3 adults in the U.S. are at risk for kidney disease. Risk factors for kidney disease include: diabetes, high blood pressure, heart disease, obesity, and family history. People of Black or African American, Hispanic or Latino, American Indian or Alaska Native, Asian American, or Native Hawaiian or Other Pacific Islander descent are at increased risk for developing the disease. Black or African American people are about four times as likely as White people to have kidney failure. Hispanics experience kidney failure at about double the rate of White people.
NKF Professional Membership
Healthcare professionals can join NKF to receive access to tools and resources for both patients and professionals, discounts on professional education, and access to a network of thousands of individuals who treat patients with kidney disease.
References
Perkovic V, Kollins D, Renfurm R, et al. Efficacy and Safety of Iptacopan in Patients with IgA Nephropathy: Interim Results from the Phase 3 APPLAUSE-IgAN Study. Presented at the World Congress of Nephrology (WCN); April 15, 2024; Buenos Aires, Argentina.Kidney Disease: Improving Global Outcomes (KDIGO) 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100(4):S1-S276. doi:10.1016/j.kint.2021.05.021Rizk DV, Maillard N, Julian BA, et al. The Emerging Role of Complement Proteins as a Target for Therapy of IgA Nephropathy. Front Immunol. 2019;10:504. doi:10.3389/fimmu.2019.00504Medjeral-Thomas NR, O’Shaughnessy MM. Complement in IgA Nephropathy: The Role of Complement in the Pathogenesis, Diagnosis, and Future Management of IgA Nephropathy. Adv Chronic Kidney Dis. 2020;27(2):111-119. doi:10.1053/j.ackd.2019.12.004Boyd JK, Cheung CK, Molyneux K, Feehally J, Barratt J. An Update on the Pathogenesis and Treatment of IgA Nephropathy. Kidney Int. 2012;81(9):833-843. doi:10.1038/ki.2011.501Reich HN, Troyanov SAA, Scholey JW, Cattran DC. Remission of Proteinuria Improves Prognosis in IgA Nephropathy. J Am Soc Nephrol. 2007;18(12):3177-3183. doi:10.1681/ASN.2007050526Thompson A, Carroll K, Inker LA, et al. Proteinuria Reduction as a Surrogate End Point in Trials of IgA Nephropathy. Clin J Am Soc Nephrol. 2019;14(3):469-481. doi:10.2215/CJN.08600718Zhang H, Rizk DV, Perkovic V, et al. Results of a Randomized Double-Blind Placebo-Controlled Phase 2 Study Propose Iptacopan as an Alternative Complement Pathway Inhibitor for IgA Nephropathy. Kidney Int. 2024;105(1):189-199. doi:10.1016/j.kint.2023.09.027Rizk DV, Rovin BH, Zhang H, et al. Targeting the Alternative Complement Pathway with Iptacopan to Treat IgA Nephropathy: Design and Rationale of the APPLAUSE-IgAN Study. Kidney Int Rep. 2023;8(5):968-979. doi:10.1016/j.ekir.2023.01.041ClinicalTrials.gov. NCT04578834. A Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Parallel Group, Phase III Study to Evaluate the Efficacy and Safety of LNP023 in Primary IgA Nephropathy Patients. Available from: https://clinicaltrials.gov/ct2/show/NCT04578834. Accessed April 2024.Novartis. Novartis receives FDA approval for Fabhalta® (iptacopan), offering superior hemoglobin improvement in the absence of transfusions as the first oral monotherapy for adults with PNH. Available from: https://www.novartis.com/news/media-releases/novartis-receives-fda-approval-fabhalta-iptacopan-offering-superior-hemoglobin-improvement-absence-transfusions-first-oral-monotherapy-adults-pnh. Accessed April 2024.Novartis. Novartis Fabhalta® (iptacopan) receives positive CHMP opinion as first oral monotherapy for adult patients with paroxysmal nocturnal hemoglobinuria (PNH). Available from: https://www.novartis.com/news/media-releases/novartis-fabhalta-iptacopan-receives-positive-chmp-opinion-first-oral-monotherapy-adult-patients-paroxysmal-nocturnal-hemoglobinuria-pnh. Accessed April 2024.McGrogan A, Franssen CF, de Vries CS. The Incidence of Primary Glomerulonephritis Worldwide: A Systematic Review of the Literature. Nephrol Dial Transplant. 2011;26(2):414-430. doi:10.1093/ndt/gfq665Xie J, Kiryluk K, Wang W, et al. Predicting Progression of IgA Nephropathy: New Clinical Progression Risk Score. PLoS ONE. 2012;7(6):e38904. doi:10.1371/journal.pone.0038904Novartis. Novartis completes acquisition of Chinook Therapeutics. Available from: https://www.novartis.com/news/media-releases/novartis-completes-acquisition-chinook-therapeutics. Accessed April 2024.
View original content to download multimedia:https://www.prnewswire.com/news-releases/the-national-kidney-foundations-2024-spring-clinical-meetings-late-breaking-presentation-on-new-therapy-shows-promising-results-for-igan-patients-302145473.html
SOURCE The National Kidney Foundation