Public advisory – APO-Mometasone nasal spray: Two lots recalled due to possible risk of infection
OTTAWA, ON, Feb. 19, 2024 /CNW/ –
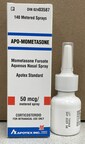
Product: APO-Mometasone nasal spray, 50 mcg/metered sprayIssue: Health products – ContaminationWhat to do: Do not use the affected product. Return it to your local pharmacy for proper disposal. Seek medical attention if you or your child have used this product and are experiencing persistent or worsening symptoms, such as fevers, or facial pain or pressure. Consult a healthcare professional if you or your child have used this product and have health concerns.
Product
DIN
Lot
Expiry
APO-Mometasone nasal spray, 50 mcg/metered spray
02403587
TX5343
TZ2586
Sep 2025
Oct 2025
Apotex Inc. is recalling two lots of APO-Mometasone nasal spray due to possible bacterial contamination with Burkholderia cepacia complex (Bcc). Bcc is a group of bacteria that poses a low medical risk to most healthy people; however, children, those with chronic lung diseases, people who are pregnant, seniors and those with a weakened immune system may be at a higher risk of illness.
APO-Mometasone nasal spray is a prescription drug used to treat seasonal and year-round nasal allergy symptoms (such as itchy, stuffy or runny nose and sneezing) in children 3-11 years old. It is also used to treat sinusitis (sinus inflammation) in adults and children 12 years of age and older, and nasal polyps in adults.
The effects of Bcc vary widely, ranging from no symptoms at all to serious infections. In severe cases, Bcc can lead to bloodstream infections that may result in sepsis (a serious medical condition caused by an overwhelming immune response to an infection) and death. Bcc is often resistant to common antibiotics. People experiencing persistent or worsening symptoms, such as fevers, or facial pain or pressure, should seek medical attention.
Health Canada is monitoring the company’s recall and its implementation of corrective and preventative actions. Supply might be temporarily constrained as a result of this recall. You should speak to your healthcare provider about what suitable alternative treatments are available to you or your child.
Do not use the affected product. Return it to your local pharmacy for proper disposal.Seek medical attention if you or your child have used this product and are experiencing persistent or worsening symptoms, such as fevers, or facial pain or pressure. Consult a health care professional if you or your child have used this product and have health concerns.Contact Apotex Inc. via Sedgwick by calling 1-844-265-7389 if you have questions about this recall.Report any health product-related side effects or complaints to Health Canada.
Également disponible en français
SOURCE Health Canada (HC)






