Nipocalimab is the first and only investigational therapy granted U.S. FDA Breakthrough Therapy Designation for the treatment of adults living with moderate-to-severe Sjögren’s disease
The Breakthrough Therapy Designation (BTD) for investigational nipocalimab in Sjögren’s disease, a prevalent autoantibody disease with no approved advanced therapies, is supported by results from the Phase 2 DAHLIAS study A greater than 70 percent relative improvement in systemic disease acti
MARPAI REPORTS THIRD QUARTER 2024 FINANCIAL RESULTS
Turnaround continues to gain traction TAMPA, Fla., Nov. 11, 2024 /PRNewswire/ — Marpai, Inc. (“Marpai” or the “Company”) (OTCQX: MRAI), a technology platform company, which operates as a national Third-Party Administrator (TPA) through its subsidiaries and is transfor
Spyre Therapeutics to Host Conference Call and Webcast to Report Interim Results from Phase 1 Healthy Volunteer Trial for SPY001, its Novel Half-Life Extended Anti-α4β7 Antibody for the Treatment for Inflammatory Bowel Disease on November 12, 2024
WALTHAM, Mass., Nov. 11, 2024 /PRNewswire/ — Spyre Therapeutics, Inc. (NASDAQ: SYRE) (the “Company” or “Spyre”), a clinical-stage biotechnology company utilizing best-in-class antibody engineering, rational therapeutic combinations, and precision medicine approaches to
Local Bounti to Release Third Quarter 2024 Financial Results on Thursday, November 14, 2024
HAMILTON, Mont., Nov. 11, 2024 /PRNewswire/ — Local Bounti Corporation (NYSE: LOCL) (“Local Bounti” or the “Company”), a breakthrough U.S. indoor agriculture company, today announced it will release its financial results for the fiscal third quarter ended September 30
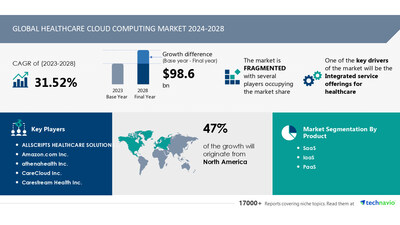
Healthcare Cloud Computing Market to Grow by USD 98.6 Billion from 2024-2028, as Integrated Services Drive Demand with AI-Powered Market Evolution – Technavio
NEW YORK, Nov. 11, 2024 /PRNewswire/ — Report on how AI is driving market transformation – The global healthcare cloud computing market size is estimated to grow by USD 98.6 billion from 2024-2028, according to Technavio. The market is estimated to grow at a CAGR of 31.52% during th
BlipCut V3.0 Launches with AI Clipping Feature to Boost Efficiency for Creators and Businesses
NEW YORK, Nov. 11, 2024 /PRNewswire/ — BlipCut, the leading AI-powered video translation platform, is excited to announce the release of its V3.0.0 update, bringing a new feature – BlipCut AI Clipping – designed to help creators as well as teams to improve the efficiency of video c
World Insurance Associates Expands Presence in Louisiana and Houston, Texas
ISELIN, N.J., Nov. 11, 2024 /PRNewswire/ — World Insurance Associates LLC (“World”), a Top 50 Insurance Brokerage, announced today that it acquired the business of five entities with locations across Louisiana and Texas on August 1, 2024. The agencies are The Firm of Louisiana P&am
Avirmax Inc. to Present at the ARVO Frontiers in Ocular Gene Therapy Research Conference
HAYWARD, Calif., Nov. 11, 2024 /PRNewswire/ — Avirmax’s Chief Executive and Scientific Officer, Dr. Shengjiang Shawn Liu, Ph.D., will chair the upcoming session of “Acceleration of AAV Gene Therapy for Ocular Diseases: Improving Delivery and Quality, at the Virtual Frontiers in Oc
Northwind Group Provides a $77.2 Million First-Mortgage Loan Secured by 167 Residential Condominiums at Vesper in Austin, TX
NEW YORK, Nov. 11, 2024 /PRNewswire/ — Northwind Group, a Manhattan-based real estate private equity firm and debt fund manager announced today that it has provided a $77.2 million first mortgage condo inventory loan through its closed-end debt fund. The loan is collateralized by 167 remaini
Bolthouse Fresh Foods™ Wins 2024 Best Sustainable Packaging Award at The Global Produce & Floral Show
Recognized for Their Commitment to Innovation in Home Compostable Packaging, Made Possible Through Columbia Packaging Group’s Sustainable Solutions. KANSAS CITY, Mo., Nov. 11, 2024 /PRNewswire/ — Bolthouse Fresh Foods™, in partnership with Columbia Packaging Group, has been awarded t