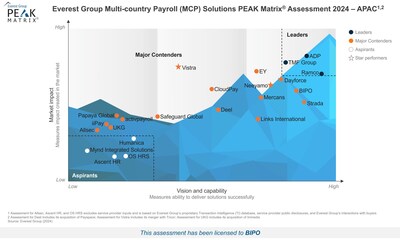
BIPO Strengthens Global HR Tech Leadership with Third Recognition in Everest Group’s PEAK Matrix® Assessment
SINGAPORE, Jan. 7, 2025 /PRNewswire/ — BIPO, a leading global HR technology and services provider, has been recognised as a Major Contender in the Everest Group Multi-Country Payroll (MCP) Solutions PEAK Matrix® Assessment 2024. This marks the third consecutive year BIPO has secured this pres
Neuraxpharm to further grow branded business with the acquisition of two leading narcolepsy treatments, Provigil® and Nuvigil®
Provigil® (modafinil) and Nuvigil® (armodafinil), two of the most trusted and widely prescribed brands in the narcolepsy category, will increase Neuraxpharm’s global CNS presence and support market entry into new territories including Australia BARCELONA, Spain and DÜSSELDORF, Germany, Jan.
zMaticoo SDK is now officially listed on Google Play SDK Index
XI’AN, China, Jan. 6, 2025 /PRNewswire/ — zMaticoo, an emerging leader of advanced programmatic advertising solutions, is proud to officially announce its recent achievement of Google SDK Index Certification. Google Play SDK Index is a platform introduced by Google to assist developers i
Vesper Bio initiates Phase Ib/IIa proof of concept study of VES001 in asymptomatic patients with gene mutations that cause frontotemporal dementia (FTD)
Dosing of VES001 has commenced in patients with causal gene mutations for FTD(GRN), a type of frontotemporal dementia which is invariably fatal This follows Vesper having received clinical trial authorisation from the Netherlands and the United Kingdom to initiate its Phase Ib/IIa ‘SORT-IN-2&#
Neuraxpharm to further grow branded business with the acquisition of two leading narcolepsy treatments, Provigil® and Nuvigil®
Provigil® (modafinil) and Nuvigil® (armodafinil), two of the most trusted and widely prescribed brands in the narcolepsy category, will increase Neuraxpharm’s global CNS presence and support market entry into new territories including Australia BARCELONA, Spain and DÜSSELDORF, Germany, Jan.
Xeltis announces completion of enrolment in EU Pivotal Trial for aXess and prepares for market approval
– Enrolment completed – 120 patients with end-stage renal disease in 22 centers in Europe– Primary endpoint readouts anticipated in Q2 2025, first market approval expected in 2026– Capability to obtain market approval is further strengthened with the appointment of Rob
Vesper Bio initiates Phase Ib/IIa proof of concept study of VES001 in asymptomatic patients with gene mutations that cause frontotemporal dementia (FTD)
Dosing of VES001 has commenced in patients with causal gene mutations for FTD(GRN), a type of frontotemporal dementia which is invariably fatal This follows Vesper having received clinical trial authorisation from the Netherlands and the United Kingdom to initiate its Phase Ib/IIa ‘SORT-IN-2&#
Xeltis announces completion of enrolment in EU Pivotal Trial for aXess and prepares for market approval
– Enrolment completed – 120 patients with end-stage renal disease in 22 centers in Europe– Primary endpoint readouts anticipated in Q2 2025, first market approval expected in 2026– Capability to obtain market approval is further strengthened with the appointment of Rob
TWSE Transforms Innovation Board into Asia’s Premier Startup Platform
TAIPEI, Jan. 7, 2025 /PRNewswire/ — The Taiwan Stock Exchange (TWSE) has unveiled its upgraded Innovation Board (Taiwan Innovation Board, TIB) on January 6, 2025, removing restrictions on qualified investors and opening the market to retail investors. This significant reform aims to boost mark
TWSE Transforms Innovation Board into Asia’s Premier Startup Platform
TAIPEI, Jan. 7, 2025 /PRNewswire/ — The Taiwan Stock Exchange (TWSE) has unveiled its upgraded Innovation Board (Taiwan Innovation Board, TIB) on January 6, 2025, removing restrictions on qualified investors and opening the market to retail investors. This significant reform aims to boost mark